FDA Proposes Withdrawal of Only Medicine Approved for Preventing Preterm Birth. What Does This Mean?
Preterm birth—the leading cause of infant mortality and disability—is a significant public health issue with only one approved clinical therapy available to help high-risk mothers carry their pregnancy to term. In light of mixed evidence on the drug’s effectiveness and an expert panel recommendation to pull the drug from the market, the future of this medicine is in limbo. To date, there has been little research on utilization rates and factors associated with its uptake and adherence. PolicyLab’s new study found that although a large number of pregnant people are eligible for this therapy, very few received the treatment and of those who did, few received the recommended number of doses. We also identified opportunities to engage high-risk pregnant patients in clinical care, such as improving patient-provider communication and reducing administrative burden.
Prevention of Preterm Birth
Preterm birth is when a baby is born too early, before 37 complete weeks of pregnancy. Complications associated with preterm birth are the leading cause of death among children under 5 years of age. The United States has one of the highest preterm birth rates in the world, with 1 of every 10 infants born preterm in 2020. More importantly, there is a pronounced, persistent racial disparity in the rate of preterm birth—African American women experience preterm birth at a rate (14.4%) about 50% higher than White women (9.1%).
Research has yet to determine the causes of preterm birth. What we do know, however, is that a history of preterm birth is the strongest clinical risk factor. Specifically, women who have had a prior preterm birth are 1.5 to 2 times more likely to have another preterm birth than those who have never had one.
17 alpha-hydroxyprogesterone caproate (“17OHPC” – brand-name “Makena”)
17OHPC—a form of progesterone administered through weekly injections—is the only pharmacotherapy approved by the Food and Drug Administration (FDA) to reduce the risk of recurrent preterm birth. The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal Fetal Medicine (SMFM) recommend that 17OHPC be offered to women with a history of preterm birth where labor began on its own (i.e., without drugs or other methods), otherwise known as spontaneous preterm birth.
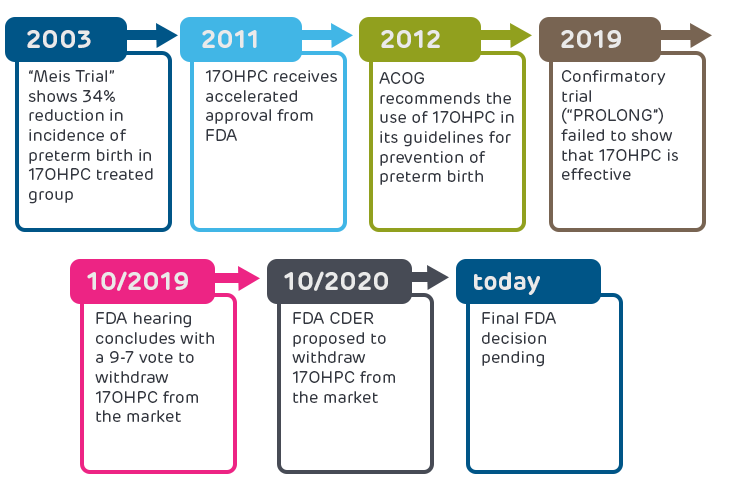
As shown in the figure below, 17OHPC was originally deemed effective after a trial found a 34% reduction in incidence of preterm birth in the treated group. FDA gave accelerated approval of 17OHPC in 2011 based on these promising results. In October 2019, the results of a required post-approval confirmatory trial (the “PROLONG trial”) were published and showed no benefit of 17OHPC. The FDA convened a hearing of researchers and health care providers to review the PROLONG trial data, and the committee concluded with a 9-7 vote to withdraw 17OHPC from the market. Following the hearing, the FDA’s Center for Drug Evaluation and Research (CDER) proposed that 17OHPC be withdrawn from the market because the PROLONG trial failed to verify clinical benefit, but the final FDA decision is still pending.
PolicyLab’s Study on 17OHPC
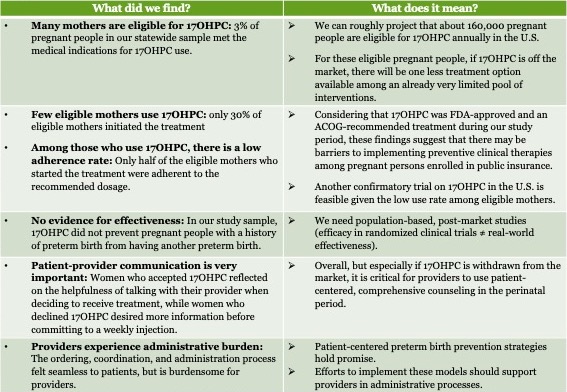
Before the PROLONG findings were released and the FDA convened its hearing, there were still many unknowns regarding how many people used 17OHPC and what contributed to their treatment decisions and adherence. PolicyLab, in partnership with the Pennsylvania Department of Health and March of Dimes, initiated a study in 2018 on the use of 17OHPC. We used linked birth certificate data and Medicaid claims in Pennsylvania to understand how many pregnant mothers, annually, were eligible for 17OHPC and how many of these women initiated and followed through with the recommended course of treatment. We also used this data to determine the sociodemographic and clinical characteristics associated with failure to initiate or complete 17OHPC therapy and the effectiveness of 17OHPC in reducing recurrent preterm births and neonatal intensive care unit admissions among Medicaid enrollees in Pennsylvania.
We simultaneously interviewed pregnant, 17OHPC-eligible women and obstetric providers to investigate the barriers and facilitators associated with 17OHPC access, administration and adherence. This work illustrated the importance of understanding 17OHPC use among eligible mothers in a large state and began to uncover the circumstances influencing their treatment-related decisions.
Our Results and Their Implications in The Current Context
This project uncovered a lot of data, and these results have significant implications in the current context of pending 17OHPC approval status. In the chart below (click to enlarge), we describe what each research finding means given 17OHPC’s unclear future.
What’s Next?
The final FDA decision is still pending. On Aug. 19, 2021, FDA granted Covis Pharmaceuticals (formerly AMAG Pharmaceuticals), the holder of 17OPHC, a public hearing to discuss 17OHPC. The hearing has not yet been scheduled, but once it occurs the FDA’s chief scientist will decide whether to withdraw approval—a process that can take months. During this time, Makena and its associated generics may remain on the market, and they represent the only medication currently available to obstetrician-gynecologists to help prevent preterm birth.
In light of the FDA’s proposal to withdraw 17OPHC from the market and its pending final decision, ACOG and the SMFM should provide updated evidence-based clinical practice guidelines on treatment for pregnant women with a history of preterm birth.*
Overall, our findings suggest there may be barriers to implementing recommended preventive clinical therapies among pregnant persons enrolled in public insurance. Further research is needed to better understand the factors influencing how a pregnant person engages with the health care system following birth and to identify effective solutions, with an eye toward remedying disparities in who receives care. For example, payers should consider financing innovative modes of treatment administration and standardized ordering processes that reduce administrative barriers for ordering clinicians.
While the future of 17OHPC is unclear, one thing is certain: effective preterm birth prevention methods are urgently needed to ensure the health and well-being of the nation’s most vulnerable citizens. Regardless of whether 17OHPC remains on the market, it is imperative that pregnant individuals at highest risk for adverse birth outcomes are prioritized within future maternal and child health efforts. PolicyLab will continue to closely follow advances in this area to promote equitable access to optimal care for pregnant people, children, and families.
*Two studies on 17OPHC were published recently. A meta-analysis published in March of 2021 reported the efficacy of various progestogens with different routes of administration (including 17OHPC) to reduce the risk of preterm birth among at-risk women. With acknowledgment of this new meta-analysis, FDA made a statement that their proposal to withdraw 17OPHC remains unchanged. In addition, a study published in November 2021 including pregnant women who received 17OHPC during pregnancy between 1959 and 1966 in California suggested that exposure to 17OHPC in the first trimester was associated with increased risk of cancers. 17OHPC was introduced in 1950s to prevent miscarriage and was used at weeks 0 through 20 weeks of pregnancy, while 17OHPC was approved by FDA in 2011 to prevent preterm birth with the treatment starting between weeks 16-20 and continuing until week 37. The new study’s implications on the current use of 17OHPC in clinical practice is unclear given the changes in indications, patient populations and administration guidelines.
Xi Wang, PhD, is a former research scientist at PolicyLab.